The lists of species containing a potential anti-addictive substance or precursor to one are just a starting point, because many of these species will probably only contain traces of the alkaloid in question and isolation of bulk quantities of the alkaloid may not be feasible. The species may also be as difficult to cultivate as iboga and be no more abundant. These details need to be worked through to identify any alkaloids and species which are more useful than what are already used.
Promising alternatives include:

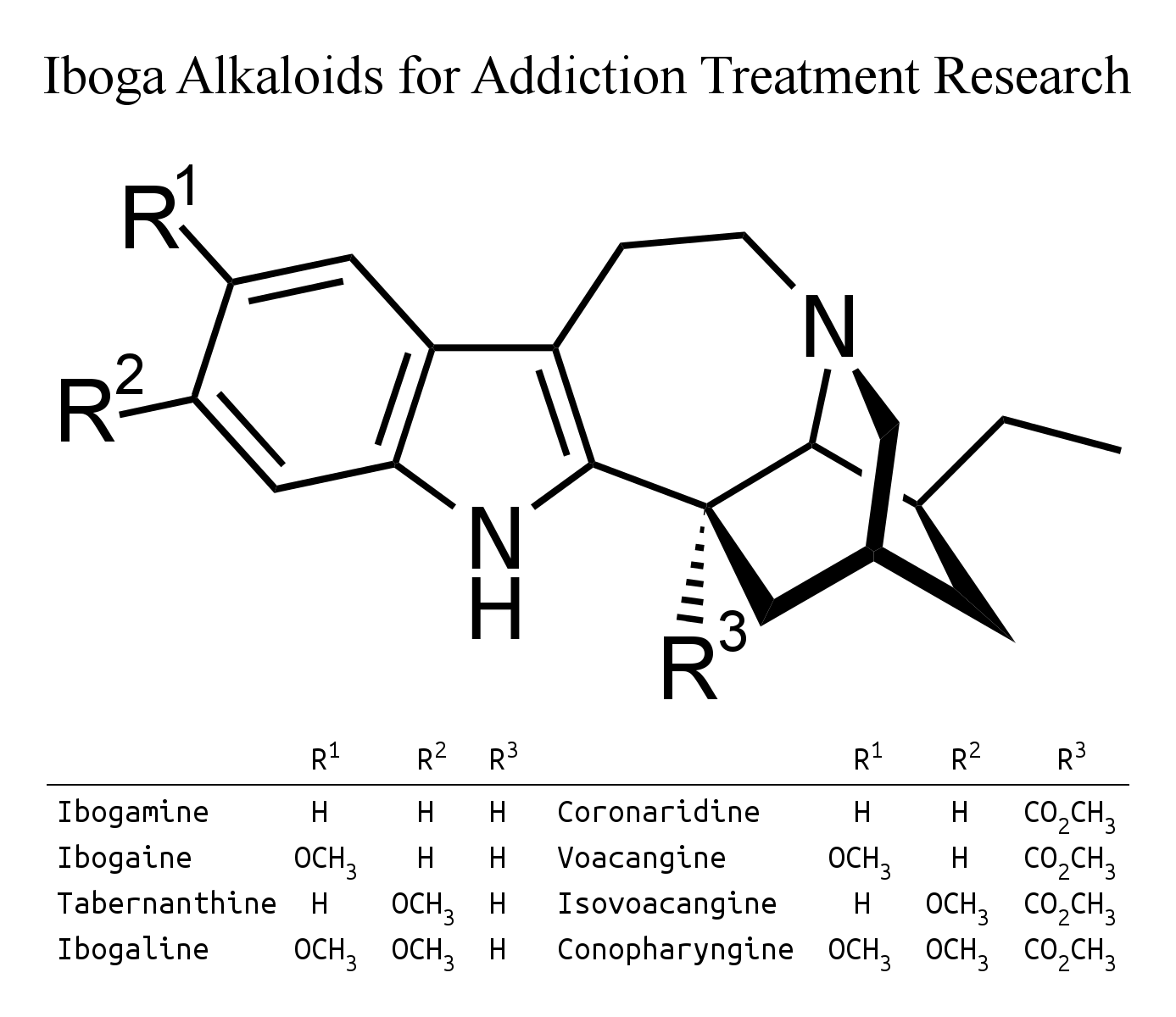
The Tabernaemontana species reported to contain ibogamine in van Beek's 1984 review were: amblyocarpa, apoda, bufalina, citrifolia, coffeoides, crassa, crassifolia, diuuricata, heyneana, olivacea, pandacaqui, quadrangularis, retusa, sananho and stapfiana.
This can be produced from coronaridine using the same process for converting voacangine into ibogaine.
"Catharanthus roseus, which is a high-yield producer of the two iboga alkaloids catharanthine and coronaridine" - "The Iboga Alkaloids>" in Progress in the Chemistry of Organic Natural Products, 105, Lavaud, 2017.
"Ervatamia divaracata. - This plant is classified by some authors as synonymous with E. coronaria and is commercially available in India. Chromatography of the alkaloidal fraction yielded coronaridine as the only homogenious material." - gorman in 1960, the paper announcing the discovery of coronaridine. However, in the experimental section it says, "Ervatamia coronaria: Coronaridine and Tabernaemontanine. - The stems (2.2 kg) were treated as described above, and the total alkaloid (8 g.) chromatographed. Elution with benzene yielded 140 mg. of coronaridine." So that's only 0.006% coronaridine. Strangely, despite calling E. divaracata and E. coronaria synonymous, it goes on to say, "E. divaracata. - Eleven kg. of stams gave 16 g. of alkaloids which yielded only coronaridine (325 mg.) on chromatography." Some predominant alkaloid - coronaridine is only 2% of the alkaloid and 0.003% of the stems.
T. psychotrifolia in the above paper had 0.32 g coronaridine (0.03%) and 0.5 g voacangine (0.04%) from 11.9 g alkaloid from 1.2 kg of ground root.
T. oppositifolia in the above paper had 300 mg ibogamine (0.025%), 70 mg coronaridine (0.006%) and 75 mg voacangine (0.006%) from 3.5 g alkaloid from 1.2 kg of ground root.
T. australis in the above paper had 1.88 g voacangine (0.05%) from 3.5 kg of stems.
T. undulata in the above paper had 1.83 g alkaloid from 1.3 kg of stems.
In the above paper, coronaridine was efficiently decarboxylated to ibogamine in 60% ethanol with potassium hydroxide.
In post: "Tabernaemontana heyneana yielded coronaridine (0.13%) (antifertility activity in female rats, estrogenic), voacangine (0.02%) (7).
Catharanthine has the structure of coronaridine with an additional, isolated double bond in the cyclohexane ring. This compound could be converted to coronaridine and then to ibogamine, or it might be decarboxylated to a new (?) alkaloid with the structure of ibogamine but including a double bond. The double bond might also be used as a means to add groups to the cyclohexane ring. The structures which come up in a google search have the opposite chirality to ibogaine, and it isn't clear whether this is an error.
From wikipedia on vinblastine:
Vinblastine was traditionally obtained from Catharanthus roseus, also known as Vinca rosea, a Madagascar Periwinkle. It is generated in the plant by the joining of two alkaloids catharanthine and vindoline.
As with the Tabernaemontana there has been a lot of confusion about the species within this genus, many also having various synonyms and type specimens. It is a mostly African and Asian genus, with only one species recorded from nth Qld, Voacanga grandifolia, for which V. papuana is a synonym. This species is recorded from New Guinea, Indonesia and the Phillipines as well. It is described as not only the most widespread Asian species, but also one of the most variable.
There is an extended discussion of the alkaloids found in this genus, including their biogenesis and pharmacology.
In one species ( V. africana ) the alkaloid content has been reported as 5-10% in root bark, 4-5% in trunk bark, 0.3-0.45% in leaves and 1.5% in seeds. From a specimen of V. grandifolia in India some indication of how the alkaloid content varied over the year was recorded, for the root and trunk bark, mar was the minimum, going up to secondary maximum in jun, then falling again in jul and peaking in nov. The leaves and fruit recorded a similar pattern, though the age of the individual leaves affected the alkaloid content. The types of alkaloids recorded was very similar to those found in Tabernanthe and Tabernaemontana. For V. grandifolia the following results of alkaloid analysis are given...
Voacanga grandifolia; synonyms V. papuana
From a cultivated specimen in India;
bark yielded 0.035% (-)-Voacangine, 0.0012% Voacamine, 0.02% Vobtusine, 0.0015% 18-oxovobtusine.
leaves yielded 0.03% Vobtusine, 0.003% 2-deoxyvobtusine, 0.0002% 18-oxovobtusine, amataine.
fruit yielded 0.004% (+)-Akuammidine; 0.0015% Tabersonine, 0.01% Vobtusine.
From New Guinea (as V. papuana);
root bark yielded 0.14% Voacangine, 0.02% Voacamine, 0.44% Vobtusine.
bark yielded 1.74% alkaloid, @ 0.2% Voacamine, 0.006% Vobtusine.
leaves yielded 0.0009% Voacamine, 0.65% Vobtusine.
fruit yielded Voacangine, traces of Voacamine, 0.52% Vobtusine.
http://www.erowid.org/plants/voacanga_africana/voacanga_africana_info1.shtml
V. africana: coronaridine analogue, eburnamine analogues, folicangine, perakine, tabersonine, voacamidine, voacamine, voacaminine, voacangine, voacarpine analogues, voacorine, voacristin, voafolidine, voafrine, voaphylline, vobtusine, yohimbine analogues
V. obtusa: vobtusine, voacangine, voacamine,
V. schweinfurthii: voacamine, voacamine analogue, voacamidine, voacangine, voacorine, vobtusine
V. thouarsii: ibogamine analogue, vobtusine analogues, voacangine, tabersonine
Characterization of V. papuana by Guise et al. also yielded the alkaloids vobtusine (77), voacamine (57) and voacangine (78).248
These compounds along with vobtusine lactone, lupeol, lupeol acetate and beta‐sitosterol were also isolated from the branch bark of V. grandifolia.249
from Identification of Novel Natural Product Antimalarial Compounds by Liza Sylvia Fernandez
"Voacanga braceata has been identified as an African botanical with hallucigenogenic potential. Stem barks of V. braceata contain 2.46% of alkaloids mainly voacamine/voacamine N-oxide, 20- epi voacamine and voacangine, although these alkaloids are related with ibogaine, it was concluded that there is no evidence of hallucinogenic properties reported De Smet (21 [De Smet, P.A.G.M., J. Ethnopharmacol. 1996, 50, 141-146])." - from http://bitnest.ca/external.php?id=%257DbxUgZ%255BCH%255E%2519vz%257F%250D%2518V%255BS%2503J%251A%252F%257Db%2515 aka koroch2009
The only Tabernaemontana species reported to contain tabernanthine in van Beek's 1984 review were: crassifolia and pandacaqui.
Tabernanthine can be produced from isovoacangine using the same process for converting voacangine into ibogaine.
Isovoacangine has been reported to be present in Stemmadenia donnell-smithii, and also, according to van Beek's 1984 review, Tabernaemontana amblyocarpa, apoda, arborea, attenuata, coffeoides, crassa, divaricata, eglandulosa, longiflora, orientalis, pandacaqui, sessilifolia, siphilitica, stapfiana and wallichiana.
Potentially from Tabernaemontana pachysiphon (T. humblotii; Giant Pinwheel Flower):
Although ibogaline is scarce in nature, with Van Beek's 1984 review mentioning it in only one Tabernaemontana species, T. humblotii leaf, more comonly known as T. pachysiphon, it should be simple to convert conopharyngine into ibogaline in the same way that voacangine is converted into ibogaine. Carroll and Starmer's 1967 paper claims that conopharyngine is "the major alkaloid present in the leaves of Tabernaemontana (Conopharyngia) pachysiphon var. cumminsi (Stapf) H. Huber" according to this reference: "THOMAS, J. & STARMER, G. A. (1963). The isolation and identification of the major alkaloid present in Tabernaemontana pachysiphon stapf var cumminsi (stapf) H. Huber. J. Pharm. Pharmac., 15, 487". However, a slightly later reference did not mention finding any conopharyngine at all: "Growth and Alkaloid Contents in Leaves of Tabernaemontana pachysiphon Stapf (Apocynaceae) as Influenced by Light Intensity, Water and Nutrient Supply, M. Hoft, R. Verpoorte and E. Beck, Oecologia, Vol. 107, No. 2 (1996), pp. 160-169". In a discouraging post, the prominent member Jacky on opiophile reported going to the extraordinary effort to have kilograms of dried leaf extracted by a chemist, who found the alkaloids scarce and laborious to process. Despite all this effort, Jacky didn't report any useful effects from the extract or up to 60 g of the leaf itself.
Other Tabernaemontana species reported to contain conopharyngine in van Beek's 1984 review were: attenuata, contorta, crassa, eglandulosa, fuchsiifolia, longiflora, orientalis and penduliflora. Though not noted among specied containing conopharyngine, Table 5 has a note saying that T. Ventricosa is "suitable for extracting conopharyngine" - which makes it sound most promising, as it may be so scarce as to only be detectable, but not isolatable, in other species. The reference (145) for this claim is: J.R. Geigy A.G. (U. Renner and D.A. Prins) (1962) Deutsche Auslegeschrift 1 129 500 (17/5/62).
Replacement of methoxy on ibogaine with ethoxy: Taylor, 1959
Conversion of ibogaine into voacangine, and related conversions: Buchi, 1966
Amperozide has shown promise in treating cocaine dependence.
If you happen to be interested and able to find any of these papers and send them to me, it would be appreciated and facilitate the above research:
Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW Jr, Carlson JN. Brain Res. 1994 Sep 19;657(1-2):14-22. Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum.
Levi MS, Borne RF. A review of chemical agents in the pharmacotherapy of addiction. Curr Med Chem. 2002 Oct;9(20):1807-18.
Abstract: Chemical substance abuse has tormented mankind throughout history. A number of chemical approaches have been employed in an attempt to treat chemical addiction. Unfortunately, most of these have proven unsuccessful though several chemical entities have been shown to be moderately effective. The naturally occurring alkaloid ibogaine has been reported to interrupt the cravings for alcohol, cocaine and opiates. Other alkaloids from Tabernanthe iboga, such as ibogamine and tabernanthine, provide insight into the structure activity relationship at the different receptors believed to be involved in addiction. The synthetic iboga alkaloid congener, 18-MC, also shows potential as an anti-addictive agent without the hallucinogenic effects of ibogaine. Additionally, acamprosate, BP 897, GBR12909, lofexidine and memantine have shown promising results in the treatment of addiction. All of these leads provide a start for the medicinal chemist to design anti-addictive agents, since currently no drugs are approved in the U.S. for the treatment of addictions to cocaine, methamphetamine, other stimulants or PCP.
Dry Column Vacuum Chromatography, D.S. Pedersen and C. Rosenbohm, Synthesis, 2001, pp. 2431-2434
^ The trick seems to be to use 1-2" of 15 - 40 micrometer silica with vacuum to make a MPLC
Vacuum Dry Column Chromatography. J. Org. Chem., 1982, 47, 4592-4594
Bandarage, U. K.; Kuehne, M. E.; Glic k, S. D., "Total Syntheses of Racemic Albifloranine and Its Anti-Addi ctive Congeners, Including 18- Methoxycoronaridine." Tetrahedron 1999, 55, (31), 9405-9424.
Bandarage, U. K.; Kuehne, M. E.; G lick, S. D., "Chemical Synthesis and Biological Evaluation of 18-Methoxycorona ridine (18-MC) as a Potential Anti- Addictive Agent." Curr. Med. Chem.: Cent. Nerv. Syst. Agents 2001, 1, (2), 113- 123.
Kuehne, M. E.; Wilson, T. E.; Ba ndarage, U. K.; Dai, W.; Yu, Q., "Enantioselective Syntheses of Cor onaridine and 18-Methoxycoronaridine." Tetrahedron 2001, 57, (11), 2085-2094.
Kuehne, M. E.; He, L.; Jokiel, P. A.; Pace, C. J.; Fleck, M. W.; Maisonneuve, I. M.; Glick, S. D.; Bidlack, J. M., "Synt hesis and Biological Evaluation of 18- Methoxycoronaridine Congeners. Potential Antiaddiction Agents." J. Med. Chem. 2003, 46, (13), 2716-2730.
Winkler, Naturwissenschaften 48, 694 (1961)